The basics
Toucan Eco uses water, salt and electricity in the form of an electrochemical reaction, which rearranges the elements by electrolysis.
This creates a reactant soup of hypochlorous acid (or HOCl), a powerful and safe disinfectant that’s also produced by the body’s immune system, along with a low concentration of sodium hypochlorite, an effective cleaner at a gentle pH.
That’s how Toucan Eco lets you create your own multipurpose disinfectant cleaner.
The solution contains 99.99% water, which shows just how reactant the active ingredients are, so much so that it is proven to have a high kill rate of up to 99.999%, with a fast contact time, minimal regrowth and good cleaning results. And, certified to EN 14476, EN 16777, EN1276 and EN13697 for chemical disinfectants.
But, we do sometimes get asked if there are ways of changing the balance of disinfecting and cleaning properties of Toucan Eco, and for more information please read on.
Out of the tap
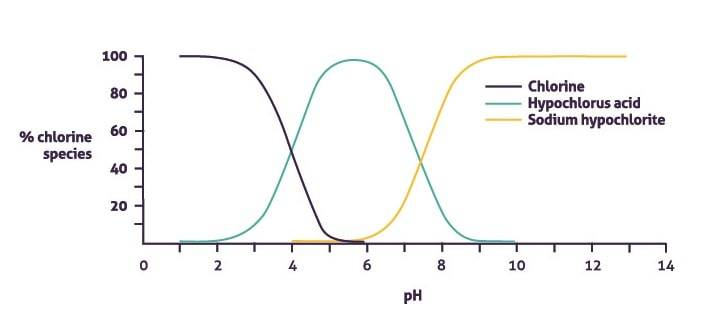
Toucan Eco at one activation typically makes 100 parts per million (ppm) of free available chlorine (FAC) – but this is dependant on the model – and the balance of hypochlorous acid and sodium hypochlorite is affected by the pH of the solution during electrolysis.
Now, tap water is typically around pH 7 to 7.5. And, once the solution is electrochemically activated, it raises the pH by around 1 point, creating a solution at a pH of between 8 to 8.5.
At this level, of the 100ppm of chlorine around 20% is hypochlorous acid and 80% is sodium hypochlorite. The chart below shows how the mix of hypochlorous and sodium hypochlorite – both chlorine species – are dependent on its pH.

Adjusting the pH of the solution
We think we’ve got this just about right but higher concentrations of hypochlorous acid can be achieved by lowering the pH of the solution so it’s more acidic, down to an optimum pH 5.5, but that means the proportion of sodium hypochlorite decreases, along with solution’s cleaning properties.
You can increase the percentage of hypochlorous acid to over 99% by adding vinegar, which is acidic and contains around 5% acetic acid. It’s safe and inexpensive, and has been used in cleaning for centuries. To do this you need to add enough vinegar to lower the pH level to around 5.5 to 6.
At pH 5.5, over 99% of free available chlorine is in the form of hypochlorous acid. This decreases to over 90% at pH 6, over 80% at pH 7, and 20% at pH 8.
As a simple guide, if using vinegar you will need to add around a teaspoon to every litre of water; that’s 6ml. If using acetic acid, then much, much less. To make sure you achieve the required level you can use pH paper or an electronic pH indicator to test the level is correct.
Now, to improve the cleaning properties of the solution, the pH can be increased so the solution is more alkaline, increasing the balance of sodium hypochlorite, but this reduces the disinfecting and deodorising properties of the solution.
You can do this by adding potassium carbonate, which is also known as potash or pearl ash, and is mainly used in the production of soap and glass. To do this, add potassium carbonate at a ratio to salt of 1:1, changing the pH to around 10 plus.
Double the dose
Two more options you have is to activate the solution twice, doubling the ppm of free available chlorine contained in the water to around 200ppm, and at the same balance of hypochlorous acid to sodium hypochlorite.
Or you can add double the amount of salt added to the water, which affects the solution in much the same way, although this is only recommended for the larger models, not Toucan Eco III.
Final points
Cleaners, who are used to diluting concentrates of cleaning chemicals, sometimes ask if this can be done with this solution.
The simple answer is ‘no’, as the tests have been done at 100ppm, although for floor cleaning we have been asked to calibrate the bucket dosing systems that are available with the larger Toucan Eco Active Plus and Flow models down to 50ppm. We wouldn’t recommend doing this for normal surface cleaning though.
If you are scientific minded and like to test how the above points affect your solution you can do this yourself in two ways.
Firstly, by using chlorine test paper that changes colour according to the ppm of the solution. Just making sure it measures the right scale – we use between 0 to 200ppm like this one here.
And secondly, by purchasing a pH test paper or an electronic pH tester, which only costs a little over £10 and is also available widely.
Finally, please bear in that, as we mentioned at the very start, the solution that’s made from tap water, salt and electricity without changing its pH has been proven to be very effective and certified as a chemical disinfectant (although we use the term ‘chemical’ loosely here as there aren’t any synthetic and dangerous chemicals included).
That’s why we think we have the balance of disinfecting and cleaning performance just about right.